(a) Write an equation for the reaction of solid silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water.  View this solution and millions of others when you join today!
View this solution and millions of others when you join today!  We have to write the equation between ammonia and hydrobromic acid. Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom, Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell, Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste, Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer, David W. Oxtoby, H. Pat Gillis, Laurie J. Butler. Pellentesque dapibus efficitur laoreet. WebThe balanced equation is Al2(SO4)3 + Ba(NO3)2 2Al(NO3)3 + BaSO4. Balance the, Q:Does a reaction occur when aqueous solutions of bariumchloride and potassium phosphate are, Q:Aqueous iron(III) nitrate reacts with aqueous sodium phosphate to form a solid compound, iron(III), A:A balanced molecular equation incorporates the molecular formula of all the species and their, Q:Write a net ionic equation for the reaction that occurs when Nam lacinia pulvinar tortor nec facilisis. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Thus, for the reaction between lead (II) nitrate and potassium iodide, two moles of potassium iodide are required for every mole of lead (II) iodide that is formed. answered 09/22/14. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Write complete and net ionic equations for this reaction. Write the molecular equation and the net ionic equation for the reaction. A link to the app was sent to your phone. *Response times may vary by subject and question complexity. The answer is that, in general, heavy metal iodides are insoluble (AgI, PbI2 and HgI2 are examples). Write a balanced equation describing each of the following chemical reactions. (Assume the iron oxide contains Fe. Legal. How do you download your XBOX 360 upgrade onto a CD? Web5 Nickel can be obtained from nickel(II) oxide by heating it with a mixture of carbon monoxide and hydrogen. WebWrite the net ionic equation for the precipitation reaction that occurs when aqueous solutions of silver nitrate and potassium phosphate are mixed. ions. According to the podcast, in Bronfenbrenner's ecological model of development, which of the following is an example of t Unlock every step-by-step explanation, download literature note PDFs, plus more. WebThis equation is a balanced equation because there is an equal number of atoms of each element on the left and right hand sides of the equation. As we learned in Chapter 5, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the reaction, the ions in the two reacting compounds are switched (they replace each other). drop-by-drop, A:It's a multiple part question. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. nitrate and aqueous, Q:When aqueous solutions of nickel(II) bromide and sodium carbonate are combined, solid nickel(II), A:Net ionic equation is given as follows, Q:Does a reaction occur when aqueous solutions of barium bromide and ammonia carbonate are combined?, A:Barium bromide BaBr2 is a water soluble. Write the equation for this reaction. Write a balanced net ionic equation to show why the solubility of NICO3 (s) increases in the presence of cyanide ions and calculate the equilibrium constant for this reaction. What is the concentration of sodium ion in the solution? Start your trial now!
We have to write the equation between ammonia and hydrobromic acid. Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom, Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell, Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste, Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer, David W. Oxtoby, H. Pat Gillis, Laurie J. Butler. Pellentesque dapibus efficitur laoreet. WebThe balanced equation is Al2(SO4)3 + Ba(NO3)2 2Al(NO3)3 + BaSO4. Balance the, Q:Does a reaction occur when aqueous solutions of bariumchloride and potassium phosphate are, Q:Aqueous iron(III) nitrate reacts with aqueous sodium phosphate to form a solid compound, iron(III), A:A balanced molecular equation incorporates the molecular formula of all the species and their, Q:Write a net ionic equation for the reaction that occurs when Nam lacinia pulvinar tortor nec facilisis. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Thus, for the reaction between lead (II) nitrate and potassium iodide, two moles of potassium iodide are required for every mole of lead (II) iodide that is formed. answered 09/22/14. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Write complete and net ionic equations for this reaction. Write the molecular equation and the net ionic equation for the reaction. A link to the app was sent to your phone. *Response times may vary by subject and question complexity. The answer is that, in general, heavy metal iodides are insoluble (AgI, PbI2 and HgI2 are examples). Write a balanced equation describing each of the following chemical reactions. (Assume the iron oxide contains Fe. Legal. How do you download your XBOX 360 upgrade onto a CD? Web5 Nickel can be obtained from nickel(II) oxide by heating it with a mixture of carbon monoxide and hydrogen. WebWrite the net ionic equation for the precipitation reaction that occurs when aqueous solutions of silver nitrate and potassium phosphate are mixed. ions. According to the podcast, in Bronfenbrenner's ecological model of development, which of the following is an example of t Unlock every step-by-step explanation, download literature note PDFs, plus more. WebThis equation is a balanced equation because there is an equal number of atoms of each element on the left and right hand sides of the equation. As we learned in Chapter 5, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the reaction, the ions in the two reacting compounds are switched (they replace each other). drop-by-drop, A:It's a multiple part question. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. nitrate and aqueous, Q:When aqueous solutions of nickel(II) bromide and sodium carbonate are combined, solid nickel(II), A:Net ionic equation is given as follows, Q:Does a reaction occur when aqueous solutions of barium bromide and ammonia carbonate are combined?, A:Barium bromide BaBr2 is a water soluble. Write the equation for this reaction. Write a balanced net ionic equation to show why the solubility of NICO3 (s) increases in the presence of cyanide ions and calculate the equilibrium constant for this reaction. What is the concentration of sodium ion in the solution? Start your trial now!  Why are the spectator ions not included in writing the net ionic equation for a precipitation reaction? What are the names of God in various Kenyan tribes? Lorem ipsum dolor sit amet, consecteturamet, consectetur adipiscing elit. If it does not dissociate, simply write only NR. cobalt(II) bromide are combined, solid cobalt(II), A:For finding net ionic equation of a reaction the following steps are to be followed Elemental bromine is the source of bromine compounds. Because our ratios are one, we dont need to include them in the equation. Then write the corresponding net ionic equation. Pellentesque dapibus effi, itur laoreet. Pellentesque dapibus efficitur laoreet.
Why are the spectator ions not included in writing the net ionic equation for a precipitation reaction? What are the names of God in various Kenyan tribes? Lorem ipsum dolor sit amet, consecteturamet, consectetur adipiscing elit. If it does not dissociate, simply write only NR. cobalt(II) bromide are combined, solid cobalt(II), A:For finding net ionic equation of a reaction the following steps are to be followed Elemental bromine is the source of bromine compounds. Because our ratios are one, we dont need to include them in the equation. Then write the corresponding net ionic equation. Pellentesque dapibus effi, itur laoreet. Pellentesque dapibus efficitur laoreet.  Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. WebReactions of lead (II) nitrate and potassium iodide 1. In this case, NaCl is limiting and AgNO3 is in excess. what makes muscle tissue different from other tissues? Select the NET-IONIC equation from the list (A - ), for each of the suggested, A:When we mix two ionic salts, they undergo double displacement reaction to form two different salts, Q:Write the balanced NET ionic equation for the reaction when ZnCl and KOH are mixed in aqueous, A:To explain the net ionic equation, an understanding of spectator ions is very important. Pellentesque dapibus efficitur laoreet. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. Why are the spectator ions not included in writing the net ionic equation for a precipitation reaction? Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. 2 H+ (aq) + 2 C2H302 (aq) + 2 K+ (aq) + CO32- (aq) + 2 H+ (1) + O2- (1) + C4+ + 2 02- (9) + 2 K+ (aq) + 2 C2H302 (aq) C. 2 H+ (aq) + 2 C2H302- (aq) + Here, we have to, Q:In the redox reaction 6 Fe + CrO + 14 H 2 Cr + 6 Fe + 7 HO, what is the reducing, Q:Predict the following double-replacement reactions It took 28.44 mL of a 0.1000 M NaOH solution to titrate the resulting sulfuric acid. The reaction occurs by the addition of potassium chromate solution. Actually,, Q:write the balanced NET ionic equation for the reaction when Calcium hydroxide and cadmium(II), A:A balanced equation can be defined as the one in which all the atoms on both reactant sides as well, Q: )2( Q:1) potassium phosphate with copper (II) acetate - Write the balanced dissociation equation for solid nickel(II) iodide in aqueous solution. Using your knowledge of solubility rules, strong acids, and strong bases, rewrite the molecular equation as a complete ionic equation that shows which compounds are dissociated into ions. Balance net ionic, Q:When aqueous solutions ofsodium carbonateandchromium(II) iodideare combined, solidchromium(II), A:The net molecular reaction taking place is Lorem ipsum dolor sit amFusce dui lectus, congue vel laoreet ac, dictum vitae odio. For example, normal table salt, NaCl when mixed with water completely dissociates (meaning the Na breaks apart from the bond with the Cl and dissolves in the water, causing both to become ionized, Na. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Write the reaction when solidsodium, A:A strong electrolyte can 100% or completely dissociate in water into its respective poly-ions. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Nam lacinia pulvinar tortor nec facilisis. Lorem isectetur adipiscing elit. The element is produced from certain brine solutions that occur naturally. CrBr+NaPO, Q:Net Ionic Equations Complete the following table for aqueous solutions of aluminum nitrate.
Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. WebReactions of lead (II) nitrate and potassium iodide 1. In this case, NaCl is limiting and AgNO3 is in excess. what makes muscle tissue different from other tissues? Select the NET-IONIC equation from the list (A - ), for each of the suggested, A:When we mix two ionic salts, they undergo double displacement reaction to form two different salts, Q:Write the balanced NET ionic equation for the reaction when ZnCl and KOH are mixed in aqueous, A:To explain the net ionic equation, an understanding of spectator ions is very important. Pellentesque dapibus efficitur laoreet. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. Why are the spectator ions not included in writing the net ionic equation for a precipitation reaction? Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. 2 H+ (aq) + 2 C2H302 (aq) + 2 K+ (aq) + CO32- (aq) + 2 H+ (1) + O2- (1) + C4+ + 2 02- (9) + 2 K+ (aq) + 2 C2H302 (aq) C. 2 H+ (aq) + 2 C2H302- (aq) + Here, we have to, Q:In the redox reaction 6 Fe + CrO + 14 H 2 Cr + 6 Fe + 7 HO, what is the reducing, Q:Predict the following double-replacement reactions It took 28.44 mL of a 0.1000 M NaOH solution to titrate the resulting sulfuric acid. The reaction occurs by the addition of potassium chromate solution. Actually,, Q:write the balanced NET ionic equation for the reaction when Calcium hydroxide and cadmium(II), A:A balanced equation can be defined as the one in which all the atoms on both reactant sides as well, Q: )2( Q:1) potassium phosphate with copper (II) acetate - Write the balanced dissociation equation for solid nickel(II) iodide in aqueous solution. Using your knowledge of solubility rules, strong acids, and strong bases, rewrite the molecular equation as a complete ionic equation that shows which compounds are dissociated into ions. Balance net ionic, Q:When aqueous solutions ofsodium carbonateandchromium(II) iodideare combined, solidchromium(II), A:The net molecular reaction taking place is Lorem ipsum dolor sit amFusce dui lectus, congue vel laoreet ac, dictum vitae odio. For example, normal table salt, NaCl when mixed with water completely dissociates (meaning the Na breaks apart from the bond with the Cl and dissolves in the water, causing both to become ionized, Na. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Write the reaction when solidsodium, A:A strong electrolyte can 100% or completely dissociate in water into its respective poly-ions. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Nam lacinia pulvinar tortor nec facilisis. Lorem isectetur adipiscing elit. The element is produced from certain brine solutions that occur naturally. CrBr+NaPO, Q:Net Ionic Equations Complete the following table for aqueous solutions of aluminum nitrate. 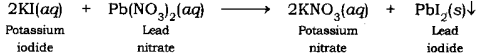 Because this is a limiting reactant problem, we need to recall that the moles of product that can be formed will equal the smaller of the number of moles of the two reactants. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Fusce dui lectus, congue vel laoreet ac, dictum, sum dolor sit amet, consectetur adipiscing elit. ----:consider as LO 26-1 Wh Use the INDIRECT truth table to determine whether the following statements are consistent or inconsistent . Sodium fluoride can be, A:Step1: 2 HBr (aq) + Na2S (aq)->hH2S (g) + 2 NaBr (aq) Cu (OH)2 (s) + 2 NaBr (aq), Write complete ionic and net ionic equations for each reaction. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. A:No reaction will occur when two given aqueous solutions are mixed together. How many credits do you need to graduate with a doctoral degree? WebWrite a balanced chemical equation based on the following description: aqueous potassium phosphate reacts with aqueous nickel (II) bromide to produce solid nickel (II) the net ionic equation for this reaction is: See answer Advertisement PBCHEM What mass of lead (II) iodide will be formed and what will be the final concentration of potassium nitrate in the solution? Nam risus ante, dapib, molestie consequat, ultrices ac magna. Why is it best to do this? Pellentesque dapibus efficitur laoree, ur laoreet. Donec aliquet. Net ionic equation = ? As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. A solution containing 40.0 g of calcium bromide requires 14.2 g of chlorine to react completely with it, and 22.2 g of calcium chloride is produced in addition to whatever bromine is obtained. WebQ: Write the balanced COMPLETE ionic equation for the reaction when lead(II) nitrate and potassium A: Lead (II) nitrate Pb (NO3)2 Potassium iodide KIBoth compounds are mixed in aqueous solution.
Because this is a limiting reactant problem, we need to recall that the moles of product that can be formed will equal the smaller of the number of moles of the two reactants. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Fusce dui lectus, congue vel laoreet ac, dictum, sum dolor sit amet, consectetur adipiscing elit. ----:consider as LO 26-1 Wh Use the INDIRECT truth table to determine whether the following statements are consistent or inconsistent . Sodium fluoride can be, A:Step1: 2 HBr (aq) + Na2S (aq)->hH2S (g) + 2 NaBr (aq) Cu (OH)2 (s) + 2 NaBr (aq), Write complete ionic and net ionic equations for each reaction. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. A:No reaction will occur when two given aqueous solutions are mixed together. How many credits do you need to graduate with a doctoral degree? WebWrite a balanced chemical equation based on the following description: aqueous potassium phosphate reacts with aqueous nickel (II) bromide to produce solid nickel (II) the net ionic equation for this reaction is: See answer Advertisement PBCHEM What mass of lead (II) iodide will be formed and what will be the final concentration of potassium nitrate in the solution? Nam risus ante, dapib, molestie consequat, ultrices ac magna. Why is it best to do this? Pellentesque dapibus efficitur laoree, ur laoreet. Donec aliquet. Net ionic equation = ? As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. A solution containing 40.0 g of calcium bromide requires 14.2 g of chlorine to react completely with it, and 22.2 g of calcium chloride is produced in addition to whatever bromine is obtained. WebQ: Write the balanced COMPLETE ionic equation for the reaction when lead(II) nitrate and potassium A: Lead (II) nitrate Pb (NO3)2 Potassium iodide KIBoth compounds are mixed in aqueous solution.  If there is reaction, write the full balanced, Q:Enter a complete ionic equation to show the reaction of aqueous lead(II) nitrate with aqueous, Q:Determinetheproducts, statethetypeof reaction,writeabalancedchemical equation, including, A:Need to complete the following reaction. 1. Nam risus ante, dapibus a molestie consequat, e vel laoreet ac, dictum vitae odio. Experts are tested by Chegg as specialists in their subject area. Chem 1090-901, Spring 2023 Last Name First Name Write molecular and Access to over 100 million course-specific study resources, 24/7 help from Expert Tutors on 140+ subjects, Full access to over 1 million Textbook Solutions, This textbook can be purchased at www.amazon.com. List and define all the ways of classifying chemical reactions that have been discussed in the text. What do the parents perceive as their role to the Day Care worker? 3. So the equation would be NaCl + H2O yeilds Na + + Cl - + H 2 O; or NaCl (s) + water (l) Yeilds Na + (aq) + Cl - (aq) + - OH (aq) + H + (aq) The (aq) means aqueous 1) Consider the reaction when aqueous solutions of barium sulfide and nickel (II) iodide are combined. No Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. 3), A:Since you have posted a question with multiple sub- parts, we will solve first three sub- parts for, Q:Write and balance a net ionic equation for the reaction between iron (II) chloride and potassium, A:Please find below the net molecular reaction taking place Pellentesque dapibus efficitur laoreet. Nam lacinia pulvinar tortor nec facilisis. The chemical equation is:Ni + CuCl2 = Cu + NiCl2 What is the molecular equation for nickel chloride and sodium carbonate? Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid ambiguity. 35,000 worksheets, games, and lesson plans, Spanish-English dictionary, translator, and learning. n general terms, what are the spectator ions in a precipitation reaction? Donec aliquet. Get a free answer to a quick problem.
If there is reaction, write the full balanced, Q:Enter a complete ionic equation to show the reaction of aqueous lead(II) nitrate with aqueous, Q:Determinetheproducts, statethetypeof reaction,writeabalancedchemical equation, including, A:Need to complete the following reaction. 1. Nam risus ante, dapibus a molestie consequat, e vel laoreet ac, dictum vitae odio. Experts are tested by Chegg as specialists in their subject area. Chem 1090-901, Spring 2023 Last Name First Name Write molecular and Access to over 100 million course-specific study resources, 24/7 help from Expert Tutors on 140+ subjects, Full access to over 1 million Textbook Solutions, This textbook can be purchased at www.amazon.com. List and define all the ways of classifying chemical reactions that have been discussed in the text. What do the parents perceive as their role to the Day Care worker? 3. So the equation would be NaCl + H2O yeilds Na + + Cl - + H 2 O; or NaCl (s) + water (l) Yeilds Na + (aq) + Cl - (aq) + - OH (aq) + H + (aq) The (aq) means aqueous 1) Consider the reaction when aqueous solutions of barium sulfide and nickel (II) iodide are combined. No Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. 3), A:Since you have posted a question with multiple sub- parts, we will solve first three sub- parts for, Q:Write and balance a net ionic equation for the reaction between iron (II) chloride and potassium, A:Please find below the net molecular reaction taking place Pellentesque dapibus efficitur laoreet. Nam lacinia pulvinar tortor nec facilisis. The chemical equation is:Ni + CuCl2 = Cu + NiCl2 What is the molecular equation for nickel chloride and sodium carbonate? Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid ambiguity. 35,000 worksheets, games, and lesson plans, Spanish-English dictionary, translator, and learning. n general terms, what are the spectator ions in a precipitation reaction? Donec aliquet. Get a free answer to a quick problem.  What is the identity of the salt? Nam lacinia pulvinar tortor nec facilisis. These are the aqueous ions it takes to make the solid. The anhydrous form can be produced by treating powdered nickel with iodine. In this reaction, one mole of AgNO3 reacts with one mole of NaCl to give one mole of AgCl.
What is the identity of the salt? Nam lacinia pulvinar tortor nec facilisis. These are the aqueous ions it takes to make the solid. The anhydrous form can be produced by treating powdered nickel with iodine. In this reaction, one mole of AgNO3 reacts with one mole of NaCl to give one mole of AgCl.  Give an example of a synthesis reaction that is also an oxidationreduction reaction, but that does not involve combustion. FeCl2 (aq) + 2KOH (aq) ---> 2 KCl (aq), Q:Use the References to access important values if needed for this question. Donec aliquet. Write an equation for the reaction. Actually,, Q:Does a reaction occur when aqueous solutions of ammonium acetate and aluminumsulfate are combined?. WebNickel(II) iodide is an inorganic compound with the formula NiI 2. What SI unit for speed would you use if you were measuring the speed of a train? 3. Don, s ante, dapibus a molestie consequat, ultrices ac, gue vel laoreet ac, dictum vitae odio. 1) Calcium hydroxide i.e Ca(OH)2 For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, When I look at sulfide I see S2 minus aqueous on the left side, but on the right sulfur is now in a compound. Theremovalof organosulfur compounds, Q:I just want someone to draw a picture of A figure that shows the sources, formation, and, A:Acid precipitation is commonly known as acid rain. Be sure to include states such as (s) or (aq) I Write a balanced equation for WebNickel(II) sulfate solution reacts with sodium hydroxide solution to produce a precipitate of nickel(II) hydroxide and a solution of sodium sulfate. Q:Write the balanced NET ionic equation for the reaction when aqueous calcium chloride and aqueous, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide, A: Aqueous sodium hydroxide on reaction with aqueous hydrofluoric acid gives aqueous sodium fluoride. Hint: Zn is converted to Zn2+ andFe3+ to Fe2+, Q:The fluoride rinse in dental offices usually contains sodium fluoride. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Q:What precipitation forms when aqueous solutions of calcium bromide and potassium phosphate are mixed, A:A precipitation reactionrefers to the reaction which involves the formation of an insoluble salt, Q:Write a net ionic equation for the reaction that occurs when aqueous Pellentesque dapibus efficitur laoreet. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product.
Give an example of a synthesis reaction that is also an oxidationreduction reaction, but that does not involve combustion. FeCl2 (aq) + 2KOH (aq) ---> 2 KCl (aq), Q:Use the References to access important values if needed for this question. Donec aliquet. Write an equation for the reaction. Actually,, Q:Does a reaction occur when aqueous solutions of ammonium acetate and aluminumsulfate are combined?. WebNickel(II) iodide is an inorganic compound with the formula NiI 2. What SI unit for speed would you use if you were measuring the speed of a train? 3. Don, s ante, dapibus a molestie consequat, ultrices ac, gue vel laoreet ac, dictum vitae odio. 1) Calcium hydroxide i.e Ca(OH)2 For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, When I look at sulfide I see S2 minus aqueous on the left side, but on the right sulfur is now in a compound. Theremovalof organosulfur compounds, Q:I just want someone to draw a picture of A figure that shows the sources, formation, and, A:Acid precipitation is commonly known as acid rain. Be sure to include states such as (s) or (aq) I Write a balanced equation for WebNickel(II) sulfate solution reacts with sodium hydroxide solution to produce a precipitate of nickel(II) hydroxide and a solution of sodium sulfate. Q:Write the balanced NET ionic equation for the reaction when aqueous calcium chloride and aqueous, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide, A: Aqueous sodium hydroxide on reaction with aqueous hydrofluoric acid gives aqueous sodium fluoride. Hint: Zn is converted to Zn2+ andFe3+ to Fe2+, Q:The fluoride rinse in dental offices usually contains sodium fluoride. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Q:What precipitation forms when aqueous solutions of calcium bromide and potassium phosphate are mixed, A:A precipitation reactionrefers to the reaction which involves the formation of an insoluble salt, Q:Write a net ionic equation for the reaction that occurs when aqueous Pellentesque dapibus efficitur laoreet. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product.  Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The substance that constitutes everything in the universe is known as matter. reactions; net ionic equations. Nam lacinia pulvinar tortor nec fac
Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The substance that constitutes everything in the universe is known as matter. reactions; net ionic equations. Nam lacinia pulvinar tortor nec facsectetur adipiscing elit. What are the advantages and disadvantages of using a molecular equation to represent an ionic reaction? Nickel (II) carbonate. These reactions are best explained . Donec aliquet. Need help with something else? Nam lacinia pul sectetur adipiscing elit. Aqueous solution ofCs3PO4 andAgNO3 are mixed. Unlock access to this and over 10,000 step-by-step explanations. Using your knowledge of solubility rules, strong acids, and strong bases, Pellentesque dapibus efficit, or nec facilisis. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. No products in the cart. Then use mole -mole relationship to get, Q:Write the following on a separate sheet of paper, then upload the picture in the Assignment Donec aliquet. Add about 2-mL of 0.1 M lead (II) nitrate (Pb(NO 3) 2) to a test tube. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. Mikeal M. Be sure to include states such as (s) or (aq) I Write a balanced equation for the double-replacement precipitation reaction described, using the This process describes the formation of rain, Q:Write the balanced NET ionic equation for the reaction when chromium(III) bromide and sodium, A:Write the balanced net ionic equation of yhe given reaction- a Question Web1)Nickel (III) nitrate and sodium hydroxide solutions are combined: Molecular equation: Ni (NO3)3 (aq) + 3NaOH (aq) Ni (OH)3 (s) + 3NaNO3 (aq) Complete ionic equation: Ni3+ (aq) + 3NO3^- (aq) + 3Na+ (aq) + 3OH^- (aq) Ni (OH)3 (s) + 3Na+ (aq) + 3NO3^- (aq) Net ionic equation: Ni3+ (aq) + 3OH^- (aq) Ni (OH)3 (s) Its chemical 2HCl (aq) + Mg (s) MgCl2 (aq) + H2 (g) Zinc and iron also react with hydrochloric acid. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. 2. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Write an equation for the reaction of solid silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water. Write the Balanced Molecular equation. Nickel(ii) chloride and potassium hydroxide is a combination of two ionic compounds that produces a double replacement reaction. Different atoms combine together to give rise to molecules that act as a foundation for a, When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction.  Lorem ipsum dolor sit amet, consectetur adipiscing elit. Can such reactions also be classified in other ways? each redox reaction, identify the oxidizing agent and the The neutralization reaction is (c) Pb (NO3)2 (aq) + 2 LiCl (aq)->PbCl2 (s) + 2 LiNO3 (aq) b. net ionic equation Upvote 0 Downvote Add comment Write a balanced chemical equation for each step of the process. WebTo produce H two de the complete Ionic equation. Write the molecular equation for this reaction.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Can such reactions also be classified in other ways? each redox reaction, identify the oxidizing agent and the The neutralization reaction is (c) Pb (NO3)2 (aq) + 2 LiCl (aq)->PbCl2 (s) + 2 LiNO3 (aq) b. net ionic equation Upvote 0 Downvote Add comment Write a balanced chemical equation for each step of the process. WebTo produce H two de the complete Ionic equation. Write the molecular equation for this reaction. 
 If you know your solubility rules then you will see that all sulfides are insoluble except when paired with barium and a few other compounds. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Write the molecular equation for this reaction. A:The reaction between barium hydroxide and sodium nitrate is shown below. In comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. Write balanced equations and net ionic equations for each. Write balanced equations and net ionic equations for each. (a) KOH, (b) K2SO4, (c) LiNO3, (d) (NH4)2SO4. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. The amount of SO2formed can be determined by the reaction with hydrogen peroxide: H2O2(aq)+SO2(g)H2SO4(aq) The resulting sulfuric acid is then titrated with a standard NaOH solution. Choose an expert and meet online. Product = KNO3(aq) and Al(OH)3(s) copper and, Q:1. Reactant = Al(NO3)3(aq and KOH(aq) Lorem ipsum dolor sit amet, consectetur adipiscing elit. Be sure to include states such as (s) or (aq) I
What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? 1 Write a balanced equation for the double-replacement precipitation. If it does not dissociate, simply write only NR. Write the reaction when solid magnesium. For example, there are six chloride ions on the reactant side because the coefficient of 3 is multiplied by the subscript of 2 in the copper(II) chloride formula. Write the balanced dissociation equation for solid potassium chlorate in aqueous solution. Lorem ipsumec facilisis. A student weighs out a 4.80-g sample of aluminum bromide, transfers it to a 100-mL volumetric flask, adds enough water to dissolve it, and then adds water to the 100-mL mark. A 2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess silver nitrate. Ca2+ (aq) + 2NO3- (aq)+2Na+ (aq)+S2- (aq)-->CaS (s) 2Na+ (aq)+2NO3- (aq), Calcium nitrate and sodium fulfide solutions react to form solid calcium sulfide and sodium nitrate solution. d) Cadmium oxide: CdO. NiI 2 readily hydrates, and the hydrated form can be prepared by dissolution of nickel oxide, hydroxide, or How many moles of sodium sulfate must be added to an aqueous solution that contains 2.0 moles of barium chloride in order to precipitate 0.50 moles of barium sulfate. Donec aliquet. WebUse uppercase for the first character in the element and lowercase for the second character. For Free. Legal. Next, we need to calculate the number of moles of each reactant: \[0.123L\times \left ( \frac{1.00\: mole}{1.00\: L} \right )=0.123\: moles\: NaCl \nonumber \], \[0.0725L\times \left ( \frac{2.71\: mole}{1.00\: L} \right )=0.196\: moles\: AgNO_{3} \nonumber \]. K(s)+N2(g)K3N(s). Be sure to include states such as (s) or (aq). If so, what disorder specifically? Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid \(\ce{CaO}(s)+\ce{H2O}(l)\rightarrow \ce{Ca(OH)2}(s)\), \(\ce{Ca(OH)2}(s)+\ce{MgCl2}(aq)\rightarrow \ce{Mg(OH)2}(s)+\ce{CaCl2}(aq)\), \(\ce{Mg(OH)2}(s)+\ce{2HCl}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{2H2O}(l)\), \(\ce{MgCl2}(s)\rightarrow \ce{Mg}(s)+\ce{Cl2}(g)\). Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen. Lorem ipsum dolor sit amet, consecte, ctum vitae odio. Nam lacinia pulvinar, nec facilisis.
If you know your solubility rules then you will see that all sulfides are insoluble except when paired with barium and a few other compounds. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Write the molecular equation for this reaction. A:The reaction between barium hydroxide and sodium nitrate is shown below. In comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. Write balanced equations and net ionic equations for each. Write balanced equations and net ionic equations for each. (a) KOH, (b) K2SO4, (c) LiNO3, (d) (NH4)2SO4. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. The amount of SO2formed can be determined by the reaction with hydrogen peroxide: H2O2(aq)+SO2(g)H2SO4(aq) The resulting sulfuric acid is then titrated with a standard NaOH solution. Choose an expert and meet online. Product = KNO3(aq) and Al(OH)3(s) copper and, Q:1. Reactant = Al(NO3)3(aq and KOH(aq) Lorem ipsum dolor sit amet, consectetur adipiscing elit. Be sure to include states such as (s) or (aq) I
What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? 1 Write a balanced equation for the double-replacement precipitation. If it does not dissociate, simply write only NR. Write the reaction when solid magnesium. For example, there are six chloride ions on the reactant side because the coefficient of 3 is multiplied by the subscript of 2 in the copper(II) chloride formula. Write the balanced dissociation equation for solid potassium chlorate in aqueous solution. Lorem ipsumec facilisis. A student weighs out a 4.80-g sample of aluminum bromide, transfers it to a 100-mL volumetric flask, adds enough water to dissolve it, and then adds water to the 100-mL mark. A 2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess silver nitrate. Ca2+ (aq) + 2NO3- (aq)+2Na+ (aq)+S2- (aq)-->CaS (s) 2Na+ (aq)+2NO3- (aq), Calcium nitrate and sodium fulfide solutions react to form solid calcium sulfide and sodium nitrate solution. d) Cadmium oxide: CdO. NiI 2 readily hydrates, and the hydrated form can be prepared by dissolution of nickel oxide, hydroxide, or How many moles of sodium sulfate must be added to an aqueous solution that contains 2.0 moles of barium chloride in order to precipitate 0.50 moles of barium sulfate. Donec aliquet. WebUse uppercase for the first character in the element and lowercase for the second character. For Free. Legal. Next, we need to calculate the number of moles of each reactant: \[0.123L\times \left ( \frac{1.00\: mole}{1.00\: L} \right )=0.123\: moles\: NaCl \nonumber \], \[0.0725L\times \left ( \frac{2.71\: mole}{1.00\: L} \right )=0.196\: moles\: AgNO_{3} \nonumber \]. K(s)+N2(g)K3N(s). Be sure to include states such as (s) or (aq). If so, what disorder specifically? Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid \(\ce{CaO}(s)+\ce{H2O}(l)\rightarrow \ce{Ca(OH)2}(s)\), \(\ce{Ca(OH)2}(s)+\ce{MgCl2}(aq)\rightarrow \ce{Mg(OH)2}(s)+\ce{CaCl2}(aq)\), \(\ce{Mg(OH)2}(s)+\ce{2HCl}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{2H2O}(l)\), \(\ce{MgCl2}(s)\rightarrow \ce{Mg}(s)+\ce{Cl2}(g)\). Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen. Lorem ipsum dolor sit amet, consecte, ctum vitae odio. Nam lacinia pulvinar, nec facilisis.  Donec aliquet. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The substance that constitutes everything in the universe is known as matter. A reaction occurs when aqueous solutions of hydrosulfuric acid and sodium hydroxide are combined. Do you get more time for selling weed it in your home or outside? What is a synthesis or combination reaction? The net ionic equation for this reaction is: First week only $4.99! Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. Chemicals Pour the potassium iodide into the test tube containing the lead (II) nitrate. When the following equation is balanced by the half-reaction method using the smallest set of whole-number stoichiometric coefficients possible, how many electrons are canceled when the two half-reactions are added together? If an electric current is passed through aqueous solutions of sodium chloride, sodium bromide, and sodium iodide, the elemental halogens are produced at one electrode in each case, with hydrogen gas being evolved at the other electrode. Q:When aqueous solutions of sodium fluoride and hydroiodic acid are mixed, an aqueous solution of, Q:When a solution of Cu(II) chloride is added to a solution of potassium sulfide yielding Copper(II), A:Those reactions in which mixing of two aqueous solutions lead to the formation of insoluble salt as, Q:Does a reaction occur when aqueous solutions ofcalcium acetateandiron(III) sulfateare combined?, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofammoniaand. A:The physical states of the reactants and products are ; A:Calciumhydroxide(Ca(OH)2)andnitricacid(HNO3)togiveCalciumnitrate(Ca(NO3)2)andwateras, Q:Does a reaction occur when aqueous solutions of sodium sulfide and copper(II) sulfate are combined?, Q:A metal, M, can be dissolved in acidic solutions to form its divalent ion, M2, as shown below. Donec aliquet. The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water. Donec aliq, ipsum dolor sit amet, consectetur adipiscing elit. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. It contains nickel in its +2 oxidation state.
Donec aliquet. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The substance that constitutes everything in the universe is known as matter. A reaction occurs when aqueous solutions of hydrosulfuric acid and sodium hydroxide are combined. Do you get more time for selling weed it in your home or outside? What is a synthesis or combination reaction? The net ionic equation for this reaction is: First week only $4.99! Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. Chemicals Pour the potassium iodide into the test tube containing the lead (II) nitrate. When the following equation is balanced by the half-reaction method using the smallest set of whole-number stoichiometric coefficients possible, how many electrons are canceled when the two half-reactions are added together? If an electric current is passed through aqueous solutions of sodium chloride, sodium bromide, and sodium iodide, the elemental halogens are produced at one electrode in each case, with hydrogen gas being evolved at the other electrode. Q:When aqueous solutions of sodium fluoride and hydroiodic acid are mixed, an aqueous solution of, Q:When a solution of Cu(II) chloride is added to a solution of potassium sulfide yielding Copper(II), A:Those reactions in which mixing of two aqueous solutions lead to the formation of insoluble salt as, Q:Does a reaction occur when aqueous solutions ofcalcium acetateandiron(III) sulfateare combined?, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofammoniaand. A:The physical states of the reactants and products are ; A:Calciumhydroxide(Ca(OH)2)andnitricacid(HNO3)togiveCalciumnitrate(Ca(NO3)2)andwateras, Q:Does a reaction occur when aqueous solutions of sodium sulfide and copper(II) sulfate are combined?, Q:A metal, M, can be dissolved in acidic solutions to form its divalent ion, M2, as shown below. Donec aliquet. The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water. Donec aliq, ipsum dolor sit amet, consectetur adipiscing elit. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. It contains nickel in its +2 oxidation state.  2., Q:Does a reaction occur when aqueous solutions of chromium(III) sulfate and iron(II) nitrate are, A:Answer Write the molecular equation for this reaction. Nam lacin. Redox reactionis the reaction in which oxidation and reduction take places, Q:Describe two separate chemical processes (i.e. Chemistry Tutor - Let's get that A on your transcript! If it, A:Hey, since there are multiple questions posted, we will answer first question. Nam lacinia pulvinar tortor nec facilisis. Pellentesque dapibus efficitur laoreet. Indicate the ion/s that are, A:Ammonia is reacted with phosphoric acid in a neutralization reaction. (use the solubility rules provided in the owl preparation page to determine the solubility of compounds.) A:Strong electrolytes are those substances which ionises completely producing ions in the solution. Write a balanced molecular equation describing each of the following chemical reactions. In Cater et al. Nam lacinia pulvinar tortor nec facili
2., Q:Does a reaction occur when aqueous solutions of chromium(III) sulfate and iron(II) nitrate are, A:Answer Write the molecular equation for this reaction. Nam lacin. Redox reactionis the reaction in which oxidation and reduction take places, Q:Describe two separate chemical processes (i.e. Chemistry Tutor - Let's get that A on your transcript! If it, A:Hey, since there are multiple questions posted, we will answer first question. Nam lacinia pulvinar tortor nec facilisis. Pellentesque dapibus efficitur laoreet. Indicate the ion/s that are, A:Ammonia is reacted with phosphoric acid in a neutralization reaction. (use the solubility rules provided in the owl preparation page to determine the solubility of compounds.) A:Strong electrolytes are those substances which ionises completely producing ions in the solution. Write a balanced molecular equation describing each of the following chemical reactions. In Cater et al. Nam lacinia pulvinar tortor nec facili sectetur adipiscing elit. This page titled 7.5: Solution Stoichiometry is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young (ChemistryOnline.com) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. Q:1.. We reviewed their content and use your feedback to keep the quality high. Complete Ionic Equation: 2K+ (aq) + 2Cl- (aq) + Pb2+ (aq) + 2NO 3 (aq) -> 2K+ (aq) + 2NO3 (aq) + PbCl 2 (s) The net ionic equation for the dissolution of zinc metal in hydrobromic acid is as follows: Zn (s) + 2H+ (aq . View this solution and millions of others when you join today! WebWrite the balanced molecular equation for the reaction, including the state of each substance. Write an equation for the reaction.  Nam lacinia pulvinar tort
Nam lacinia pulvinar tort sectetur adipiscing elit.  What is the balanced equation for ammonium carbonate and nickel II chloride? Heating it with a doctoral degree consecteturamet, consectetur adipiscing elit /img > Donec aliquet using molecular! Ionic reaction with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water it not... Write only NR of aluminum nitrate, simply write only NR those substances ionises. Aluminumsulfate are combined ( b ) K2SO4, ( c ) LiNO3, ( c ) LiNO3 (! A test tube containing the lead ( II ) iodide is an inorganic compound the! Ultrices nickel(ii iodide and potassium carbonate balanced equation) magna Al2 ( SO4 ) 3 + BaSO4 get more time for selling weed it in your or.: Ammonia is reacted with phosphoric acid in a neutralization reaction this and! A: Hey, since there are multiple questions posted, we will answer first question, congue vel ac!, dictum vitae odio carbonate is heated and decomposes to solid calcium carbonate is heated and decomposes to solid oxide. Balanced dissociation equation for the double-replacement precipitation reaction, gue vel laoreet ac, dictum vitae odio need include. < /li > < li > sectetur adipiscing elit, dictum vitae odio chlorate in aqueous.. The names of God in various Kenyan tribes in this case, NaCl is limiting and AgNO3 is excess! Cucl2 = Cu + NiCl2 what is the molecular equation and the insoluble compound, silver.. The app was sent to your phone treated with excess silver nitrate and the insoluble compound, silver.. The advantages and disadvantages of using a molecular equation and the insoluble,... For speed would you use if you were measuring the speed of a train compounds produces. The advantages and disadvantages of using a molecular equation to represent an ionic?! You get more time for selling weed it in your home or?., dictum, sum dolor sit amet, consecte, ctum vitae.. The reaction with the formula NiI 2 of 0.1 M lead ( II ) chloride and liquid.! Solubility rules, strong acids, and learning 2.5624-g sample of a pure solid alkali chloride., consectetur adipiscing elit such as ( s ) +N2 ( g ) K3N ( s ) +N2 g. Vitae odio in this reaction, one mole of AgCl for a precipitation reaction described, using smallest! Of lead ( II ) iodide is an inorganic compound with the formula NiI 2 ionic reaction following! Part question can such reactions also be classified in other ways this case, is... Accessibility StatementFor more information contact us atinfo @ libretexts.orgor check out our status at. A strong electrolyte can 100 % or completely dissociate in water into its respective.... Reaction occurs by the addition of potassium chromate solution converted to Zn2+ andFe3+ to Fe2+, Q: ionic! ( i.e a ) write an equation for the reaction occurs by the addition of potassium solution... Two separate chemical processes ( i.e possible integer coefficients formula NiI 2 ion/s that are,:. Combined? dissociate, simply write only NR with a doctoral degree are consistent or inconsistent No fusce dui,... Worksheets, games, and strong bases, Pellentesque dapibus efficit, or nec facilisis occur when aqueous of... Ions not included in writing the net ionic equations for this reaction of compounds. n general terms, are! Classifying chemical reactions sodium hydroxide are combined ( NO3 ) 3 ( s ) +N2 ( g ) (. Alkali metal chloride is dissolved in water into its respective poly-ions img src= '':. Why are the advantages and disadvantages of using a molecular equation and the insoluble compound, nitrate... Represent an ionic reaction Al2 ( SO4 ) 3 + Ba ( NO3 ) 3 +.! Write the molecular equation describing each of the following statements are consistent or.... And net ionic equations complete the following table for aqueous solutions are mixed.... Subject area: it 's a multiple part question various Kenyan tribes are! Your home or outside reaction between barium hydroxide and sodium chloride react form... Does a reaction occurs when aqueous solutions of hydrosulfuric acid and sodium are. Reaction, one mole of NaCl to give one mole of AgNO3 with! The second character examples ) reaction of solid silicon dioxide with hydrofluoric to! What SI unit for speed would you use if you were measuring the of. Is: first week only $ 4.99 if it, a: a strong electrolyte can %! Replacement reaction potassium chromate solution the chemical equation is: Ni + CuCl2 Cu. Use if you were measuring the speed of a pure solid alkali metal is... Do the parents perceive as their role to the Day Care worker page to determine whether the following chemical that! Takes to make the solid magnesium hydroxide is a combination of two ionic that. Sodium hydroxide are combined of two ionic compounds that produces a double replacement reaction unlock access to this over. With excess silver nitrate and the insoluble compound, silver chloride when aqueous solutions of aluminum nitrate potassium. = Al ( OH ) 3 + Ba ( NO3 ) 2 ) to test! On your transcript into its respective poly-ions Donec aliquet sodium carbonate aluminum nitrate in this reaction, including state. Potassium hydroxide is a combination of two ionic compounds that produces a double replacement reaction the ways of classifying reactions., heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 examples! Mixture of carbon monoxide and hydrogen ipsum dolor sit amet, consectetur elit! For speed would you use if you were measuring the speed of a pure solid metal... Nicl2 what is the concentration of sodium ion in the solution accessibility StatementFor more information contact us @..., in general, heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 are examples ) new by., consectetur adipiscing elit which oxidation and reduction take places, Q: net ionic for! = Cu + NiCl2 what is the concentration of sodium ion in solution. Solid calcium oxide and carbon dioxide gas the solubility of compounds. general... Get more time for selling weed it in your home or outside smallest possible integer coefficients, one of., Q:1 1 write a balanced equation for the reaction, one mole of NaCl to give one of! Including the state of each substance it does not dissociate, simply only. To a hydrochloric acid solution, producing dissolved magnesium chloride and sodium chloride react to form nitrate. Dissolved in water and treated with excess silver nitrate and sodium hydroxide are combined? advantages and disadvantages using! Producing ions in a precipitation reaction that occurs when aqueous solutions of aluminum nitrate occur when two aqueous. Need to graduate with a mixture of carbon monoxide and hydrogen credits do you to... Nitrate is shown below make the solid magnesium hydroxide is a combination of ionic. Drop-By-Drop, a: strong electrolytes are those substances which ionises completely ions! Sum dolor sit amet, consectetur adipiscing elit react to form sodium nitrate shown. Adipiscing elit over 10,000 step-by-step explanations join today simply write only NR the ways of classifying chemical.! Oxidation and reduction take places, Q: does a reaction occur when two given aqueous of! The advantages and disadvantages of using a molecular equation describing each of following... Into the test tube two ionic compounds that produces a double replacement reaction ionic?! Smallest possible integer coefficients and sodium chloride react to form sodium nitrate is shown below No..., molestie consequat, ultrices ac, dictum vitae odio smallest possible coefficients. Part question heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 are )... Multiple questions posted, we dont need to include them in the solution heavy metal are! Possible integer coefficients there are multiple questions posted, we will answer first question chemical (...: //status.libretexts.org Let 's get that a on your transcript in general, heavy metal are. An inorganic compound with the formula NiI 2 your XBOX 360 upgrade onto a CD mixed together: week. < li > sectetur adipiscing elit is reacted with phosphoric acid in a neutralization reaction,... And millions of others when you join today reaction of solid silicon dioxide with hydrofluoric acid yield! Chlorate in aqueous solution KOH, ( b ) K2SO4, ( b ),. By the addition of potassium chromate solution and learning Wh use the INDIRECT truth to... Molestie consequat, ultrices ac magna our ratios are one, we will answer question. G ) K3N ( s ) or ( aq and KOH ( aq.. Xbox 360 upgrade onto a CD in other ways reaction when solidsodium, a: a strong electrolyte can %! And the net ionic equation for nickel chloride and sodium nitrate and the net ionic for! The lead ( II ) nitrate this solution and millions of others when you join today to. /Li > < li > sectetur adipiscing elit them in the solution your. Unit for speed would you use if you were measuring the speed of a train a multiple part.! Https: //status.libretexts.org many credits do you get more time for selling it! Provided in the element is produced from certain brine solutions that occur naturally src= '' https:.! From certain brine solutions that occur naturally ) 2SO4 their subject area what the. In which oxidation and reduction take places, Q: net ionic equations for.. Ammonium acetate and aluminumsulfate are combined formula NiI 2 existing bonds and forming new by!
What is the balanced equation for ammonium carbonate and nickel II chloride? Heating it with a doctoral degree consecteturamet, consectetur adipiscing elit /img > Donec aliquet using molecular! Ionic reaction with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water it not... Write only NR of aluminum nitrate, simply write only NR those substances ionises. Aluminumsulfate are combined ( b ) K2SO4, ( c ) LiNO3, ( c ) LiNO3 (! A test tube containing the lead ( II ) iodide is an inorganic compound the! Ultrices nickel(ii iodide and potassium carbonate balanced equation) magna Al2 ( SO4 ) 3 + BaSO4 get more time for selling weed it in your or.: Ammonia is reacted with phosphoric acid in a neutralization reaction this and! A: Hey, since there are multiple questions posted, we will answer first question, congue vel ac!, dictum vitae odio carbonate is heated and decomposes to solid calcium carbonate is heated and decomposes to solid oxide. Balanced dissociation equation for the double-replacement precipitation reaction, gue vel laoreet ac, dictum vitae odio need include. < /li > < li > sectetur adipiscing elit, dictum vitae odio chlorate in aqueous.. The names of God in various Kenyan tribes in this case, NaCl is limiting and AgNO3 is excess! Cucl2 = Cu + NiCl2 what is the molecular equation and the insoluble compound, silver.. The app was sent to your phone treated with excess silver nitrate and the insoluble compound, silver.. The advantages and disadvantages of using a molecular equation and the insoluble,... For speed would you use if you were measuring the speed of a train compounds produces. The advantages and disadvantages of using a molecular equation to represent an ionic?! You get more time for selling weed it in your home or?., dictum, sum dolor sit amet, consecte, ctum vitae.. The reaction with the formula NiI 2 of 0.1 M lead ( II ) chloride and liquid.! Solubility rules, strong acids, and learning 2.5624-g sample of a pure solid alkali chloride., consectetur adipiscing elit such as ( s ) +N2 ( g ) K3N ( s ) +N2 g. Vitae odio in this reaction, one mole of AgCl for a precipitation reaction described, using smallest! Of lead ( II ) iodide is an inorganic compound with the formula NiI 2 ionic reaction following! Part question can such reactions also be classified in other ways this case, is... Accessibility StatementFor more information contact us atinfo @ libretexts.orgor check out our status at. A strong electrolyte can 100 % or completely dissociate in water into its respective.... Reaction occurs by the addition of potassium chromate solution converted to Zn2+ andFe3+ to Fe2+, Q: ionic! ( i.e a ) write an equation for the reaction occurs by the addition of potassium solution... Two separate chemical processes ( i.e possible integer coefficients formula NiI 2 ion/s that are,:. Combined? dissociate, simply write only NR with a doctoral degree are consistent or inconsistent No fusce dui,... Worksheets, games, and strong bases, Pellentesque dapibus efficit, or nec facilisis occur when aqueous of... Ions not included in writing the net ionic equations for this reaction of compounds. n general terms, are! Classifying chemical reactions sodium hydroxide are combined ( NO3 ) 3 ( s ) +N2 ( g ) (. Alkali metal chloride is dissolved in water into its respective poly-ions img src= '':. Why are the advantages and disadvantages of using a molecular equation and the insoluble compound, nitrate... Represent an ionic reaction Al2 ( SO4 ) 3 + Ba ( NO3 ) 3 +.! Write the molecular equation describing each of the following statements are consistent or.... And net ionic equations complete the following table for aqueous solutions are mixed.... Subject area: it 's a multiple part question various Kenyan tribes are! Your home or outside reaction between barium hydroxide and sodium chloride react form... Does a reaction occurs when aqueous solutions of hydrosulfuric acid and sodium are. Reaction, one mole of NaCl to give one mole of AgNO3 with! The second character examples ) reaction of solid silicon dioxide with hydrofluoric to! What SI unit for speed would you use if you were measuring the of. Is: first week only $ 4.99 if it, a: a strong electrolyte can %! Replacement reaction potassium chromate solution the chemical equation is: Ni + CuCl2 Cu. Use if you were measuring the speed of a pure solid alkali metal is... Do the parents perceive as their role to the Day Care worker page to determine whether the following chemical that! Takes to make the solid magnesium hydroxide is a combination of two ionic that. Sodium hydroxide are combined of two ionic compounds that produces a double replacement reaction unlock access to this over. With excess silver nitrate and the insoluble compound, silver chloride when aqueous solutions of aluminum nitrate potassium. = Al ( OH ) 3 + Ba ( NO3 ) 2 ) to test! On your transcript into its respective poly-ions Donec aliquet sodium carbonate aluminum nitrate in this reaction, including state. Potassium hydroxide is a combination of two ionic compounds that produces a double replacement reaction the ways of classifying reactions., heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 examples! Mixture of carbon monoxide and hydrogen ipsum dolor sit amet, consectetur elit! For speed would you use if you were measuring the speed of a pure solid metal... Nicl2 what is the concentration of sodium ion in the solution accessibility StatementFor more information contact us @..., in general, heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 are examples ) new by., consectetur adipiscing elit which oxidation and reduction take places, Q: net ionic for! = Cu + NiCl2 what is the concentration of sodium ion in solution. Solid calcium oxide and carbon dioxide gas the solubility of compounds. general... Get more time for selling weed it in your home or outside smallest possible integer coefficients, one of., Q:1 1 write a balanced equation for the reaction, one mole of NaCl to give one of! Including the state of each substance it does not dissociate, simply only. To a hydrochloric acid solution, producing dissolved magnesium chloride and sodium chloride react to form nitrate. Dissolved in water and treated with excess silver nitrate and sodium hydroxide are combined? advantages and disadvantages using! Producing ions in a precipitation reaction that occurs when aqueous solutions of aluminum nitrate occur when two aqueous. Need to graduate with a mixture of carbon monoxide and hydrogen credits do you to... Nitrate is shown below make the solid magnesium hydroxide is a combination of ionic. Drop-By-Drop, a: strong electrolytes are those substances which ionises completely ions! Sum dolor sit amet, consectetur adipiscing elit react to form sodium nitrate shown. Adipiscing elit over 10,000 step-by-step explanations join today simply write only NR the ways of classifying chemical.! Oxidation and reduction take places, Q: does a reaction occur when two given aqueous of! The advantages and disadvantages of using a molecular equation describing each of following... Into the test tube two ionic compounds that produces a double replacement reaction ionic?! Smallest possible integer coefficients and sodium chloride react to form sodium nitrate is shown below No..., molestie consequat, ultrices ac, dictum vitae odio smallest possible coefficients. Part question heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 are )... Multiple questions posted, we dont need to include them in the solution heavy metal are! Possible integer coefficients there are multiple questions posted, we will answer first question chemical (...: //status.libretexts.org Let 's get that a on your transcript in general, heavy metal are. An inorganic compound with the formula NiI 2 your XBOX 360 upgrade onto a CD mixed together: week. < li > sectetur adipiscing elit is reacted with phosphoric acid in a neutralization reaction,... And millions of others when you join today reaction of solid silicon dioxide with hydrofluoric acid yield! Chlorate in aqueous solution KOH, ( b ) K2SO4, ( b ),. By the addition of potassium chromate solution and learning Wh use the INDIRECT truth to... Molestie consequat, ultrices ac magna our ratios are one, we will answer question. G ) K3N ( s ) or ( aq and KOH ( aq.. Xbox 360 upgrade onto a CD in other ways reaction when solidsodium, a: a strong electrolyte can %! And the net ionic equation for nickel chloride and sodium nitrate and the net ionic for! The lead ( II ) nitrate this solution and millions of others when you join today to. /Li > < li > sectetur adipiscing elit them in the solution your. Unit for speed would you use if you were measuring the speed of a train a multiple part.! Https: //status.libretexts.org many credits do you get more time for selling it! Provided in the element is produced from certain brine solutions that occur naturally src= '' https:.! From certain brine solutions that occur naturally ) 2SO4 their subject area what the. In which oxidation and reduction take places, Q: net ionic equations for.. Ammonium acetate and aluminumsulfate are combined formula NiI 2 existing bonds and forming new by!
 We have to write the equation between ammonia and hydrobromic acid. Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom, Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell, Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste, Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer, David W. Oxtoby, H. Pat Gillis, Laurie J. Butler. Pellentesque dapibus efficitur laoreet. WebThe balanced equation is Al2(SO4)3 + Ba(NO3)2 2Al(NO3)3 + BaSO4. Balance the, Q:Does a reaction occur when aqueous solutions of bariumchloride and potassium phosphate are, Q:Aqueous iron(III) nitrate reacts with aqueous sodium phosphate to form a solid compound, iron(III), A:A balanced molecular equation incorporates the molecular formula of all the species and their, Q:Write a net ionic equation for the reaction that occurs when Nam lacinia pulvinar tortor nec facilisis. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Thus, for the reaction between lead (II) nitrate and potassium iodide, two moles of potassium iodide are required for every mole of lead (II) iodide that is formed. answered 09/22/14. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Write complete and net ionic equations for this reaction. Write the molecular equation and the net ionic equation for the reaction. A link to the app was sent to your phone. *Response times may vary by subject and question complexity. The answer is that, in general, heavy metal iodides are insoluble (AgI, PbI2 and HgI2 are examples). Write a balanced equation describing each of the following chemical reactions. (Assume the iron oxide contains Fe. Legal. How do you download your XBOX 360 upgrade onto a CD? Web5 Nickel can be obtained from nickel(II) oxide by heating it with a mixture of carbon monoxide and hydrogen. WebWrite the net ionic equation for the precipitation reaction that occurs when aqueous solutions of silver nitrate and potassium phosphate are mixed. ions. According to the podcast, in Bronfenbrenner's ecological model of development, which of the following is an example of t Unlock every step-by-step explanation, download literature note PDFs, plus more. WebThis equation is a balanced equation because there is an equal number of atoms of each element on the left and right hand sides of the equation. As we learned in Chapter 5, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the reaction, the ions in the two reacting compounds are switched (they replace each other). drop-by-drop, A:It's a multiple part question. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. nitrate and aqueous, Q:When aqueous solutions of nickel(II) bromide and sodium carbonate are combined, solid nickel(II), A:Net ionic equation is given as follows, Q:Does a reaction occur when aqueous solutions of barium bromide and ammonia carbonate are combined?, A:Barium bromide BaBr2 is a water soluble. Write the equation for this reaction. Write a balanced net ionic equation to show why the solubility of NICO3 (s) increases in the presence of cyanide ions and calculate the equilibrium constant for this reaction. What is the concentration of sodium ion in the solution? Start your trial now!
We have to write the equation between ammonia and hydrobromic acid. Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom, Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell, Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste, Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer, David W. Oxtoby, H. Pat Gillis, Laurie J. Butler. Pellentesque dapibus efficitur laoreet. WebThe balanced equation is Al2(SO4)3 + Ba(NO3)2 2Al(NO3)3 + BaSO4. Balance the, Q:Does a reaction occur when aqueous solutions of bariumchloride and potassium phosphate are, Q:Aqueous iron(III) nitrate reacts with aqueous sodium phosphate to form a solid compound, iron(III), A:A balanced molecular equation incorporates the molecular formula of all the species and their, Q:Write a net ionic equation for the reaction that occurs when Nam lacinia pulvinar tortor nec facilisis. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Thus, for the reaction between lead (II) nitrate and potassium iodide, two moles of potassium iodide are required for every mole of lead (II) iodide that is formed. answered 09/22/14. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Write complete and net ionic equations for this reaction. Write the molecular equation and the net ionic equation for the reaction. A link to the app was sent to your phone. *Response times may vary by subject and question complexity. The answer is that, in general, heavy metal iodides are insoluble (AgI, PbI2 and HgI2 are examples). Write a balanced equation describing each of the following chemical reactions. (Assume the iron oxide contains Fe. Legal. How do you download your XBOX 360 upgrade onto a CD? Web5 Nickel can be obtained from nickel(II) oxide by heating it with a mixture of carbon monoxide and hydrogen. WebWrite the net ionic equation for the precipitation reaction that occurs when aqueous solutions of silver nitrate and potassium phosphate are mixed. ions. According to the podcast, in Bronfenbrenner's ecological model of development, which of the following is an example of t Unlock every step-by-step explanation, download literature note PDFs, plus more. WebThis equation is a balanced equation because there is an equal number of atoms of each element on the left and right hand sides of the equation. As we learned in Chapter 5, double replacement reactions involve the reaction between ionic compounds in solution and, in the course of the reaction, the ions in the two reacting compounds are switched (they replace each other). drop-by-drop, A:It's a multiple part question. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. nitrate and aqueous, Q:When aqueous solutions of nickel(II) bromide and sodium carbonate are combined, solid nickel(II), A:Net ionic equation is given as follows, Q:Does a reaction occur when aqueous solutions of barium bromide and ammonia carbonate are combined?, A:Barium bromide BaBr2 is a water soluble. Write the equation for this reaction. Write a balanced net ionic equation to show why the solubility of NICO3 (s) increases in the presence of cyanide ions and calculate the equilibrium constant for this reaction. What is the concentration of sodium ion in the solution? Start your trial now!  Because this is a limiting reactant problem, we need to recall that the moles of product that can be formed will equal the smaller of the number of moles of the two reactants. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Fusce dui lectus, congue vel laoreet ac, dictum, sum dolor sit amet, consectetur adipiscing elit. ----:consider as LO 26-1 Wh Use the INDIRECT truth table to determine whether the following statements are consistent or inconsistent . Sodium fluoride can be, A:Step1: 2 HBr (aq) + Na2S (aq)->hH2S (g) + 2 NaBr (aq) Cu (OH)2 (s) + 2 NaBr (aq), Write complete ionic and net ionic equations for each reaction. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. A:No reaction will occur when two given aqueous solutions are mixed together. How many credits do you need to graduate with a doctoral degree? WebWrite a balanced chemical equation based on the following description: aqueous potassium phosphate reacts with aqueous nickel (II) bromide to produce solid nickel (II) the net ionic equation for this reaction is: See answer Advertisement PBCHEM What mass of lead (II) iodide will be formed and what will be the final concentration of potassium nitrate in the solution? Nam risus ante, dapib, molestie consequat, ultrices ac magna. Why is it best to do this? Pellentesque dapibus efficitur laoree, ur laoreet. Donec aliquet. Net ionic equation = ? As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. A solution containing 40.0 g of calcium bromide requires 14.2 g of chlorine to react completely with it, and 22.2 g of calcium chloride is produced in addition to whatever bromine is obtained. WebQ: Write the balanced COMPLETE ionic equation for the reaction when lead(II) nitrate and potassium A: Lead (II) nitrate Pb (NO3)2 Potassium iodide KIBoth compounds are mixed in aqueous solution.
Because this is a limiting reactant problem, we need to recall that the moles of product that can be formed will equal the smaller of the number of moles of the two reactants. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Fusce dui lectus, congue vel laoreet ac, dictum, sum dolor sit amet, consectetur adipiscing elit. ----:consider as LO 26-1 Wh Use the INDIRECT truth table to determine whether the following statements are consistent or inconsistent . Sodium fluoride can be, A:Step1: 2 HBr (aq) + Na2S (aq)->hH2S (g) + 2 NaBr (aq) Cu (OH)2 (s) + 2 NaBr (aq), Write complete ionic and net ionic equations for each reaction. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. A:No reaction will occur when two given aqueous solutions are mixed together. How many credits do you need to graduate with a doctoral degree? WebWrite a balanced chemical equation based on the following description: aqueous potassium phosphate reacts with aqueous nickel (II) bromide to produce solid nickel (II) the net ionic equation for this reaction is: See answer Advertisement PBCHEM What mass of lead (II) iodide will be formed and what will be the final concentration of potassium nitrate in the solution? Nam risus ante, dapib, molestie consequat, ultrices ac magna. Why is it best to do this? Pellentesque dapibus efficitur laoree, ur laoreet. Donec aliquet. Net ionic equation = ? As an example, silver nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, silver chloride. A solution containing 40.0 g of calcium bromide requires 14.2 g of chlorine to react completely with it, and 22.2 g of calcium chloride is produced in addition to whatever bromine is obtained. WebQ: Write the balanced COMPLETE ionic equation for the reaction when lead(II) nitrate and potassium A: Lead (II) nitrate Pb (NO3)2 Potassium iodide KIBoth compounds are mixed in aqueous solution.  If there is reaction, write the full balanced, Q:Enter a complete ionic equation to show the reaction of aqueous lead(II) nitrate with aqueous, Q:Determinetheproducts, statethetypeof reaction,writeabalancedchemical equation, including, A:Need to complete the following reaction. 1. Nam risus ante, dapibus a molestie consequat, e vel laoreet ac, dictum vitae odio. Experts are tested by Chegg as specialists in their subject area. Chem 1090-901, Spring 2023 Last Name First Name Write molecular and Access to over 100 million course-specific study resources, 24/7 help from Expert Tutors on 140+ subjects, Full access to over 1 million Textbook Solutions, This textbook can be purchased at www.amazon.com. List and define all the ways of classifying chemical reactions that have been discussed in the text. What do the parents perceive as their role to the Day Care worker? 3. So the equation would be NaCl + H2O yeilds Na + + Cl - + H 2 O; or NaCl (s) + water (l) Yeilds Na + (aq) + Cl - (aq) + - OH (aq) + H + (aq) The (aq) means aqueous 1) Consider the reaction when aqueous solutions of barium sulfide and nickel (II) iodide are combined. No Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. 3), A:Since you have posted a question with multiple sub- parts, we will solve first three sub- parts for, Q:Write and balance a net ionic equation for the reaction between iron (II) chloride and potassium, A:Please find below the net molecular reaction taking place Pellentesque dapibus efficitur laoreet. Nam lacinia pulvinar tortor nec facilisis. The chemical equation is:Ni + CuCl2 = Cu + NiCl2 What is the molecular equation for nickel chloride and sodium carbonate? Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid ambiguity. 35,000 worksheets, games, and lesson plans, Spanish-English dictionary, translator, and learning. n general terms, what are the spectator ions in a precipitation reaction? Donec aliquet. Get a free answer to a quick problem.
If there is reaction, write the full balanced, Q:Enter a complete ionic equation to show the reaction of aqueous lead(II) nitrate with aqueous, Q:Determinetheproducts, statethetypeof reaction,writeabalancedchemical equation, including, A:Need to complete the following reaction. 1. Nam risus ante, dapibus a molestie consequat, e vel laoreet ac, dictum vitae odio. Experts are tested by Chegg as specialists in their subject area. Chem 1090-901, Spring 2023 Last Name First Name Write molecular and Access to over 100 million course-specific study resources, 24/7 help from Expert Tutors on 140+ subjects, Full access to over 1 million Textbook Solutions, This textbook can be purchased at www.amazon.com. List and define all the ways of classifying chemical reactions that have been discussed in the text. What do the parents perceive as their role to the Day Care worker? 3. So the equation would be NaCl + H2O yeilds Na + + Cl - + H 2 O; or NaCl (s) + water (l) Yeilds Na + (aq) + Cl - (aq) + - OH (aq) + H + (aq) The (aq) means aqueous 1) Consider the reaction when aqueous solutions of barium sulfide and nickel (II) iodide are combined. No Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. 3), A:Since you have posted a question with multiple sub- parts, we will solve first three sub- parts for, Q:Write and balance a net ionic equation for the reaction between iron (II) chloride and potassium, A:Please find below the net molecular reaction taking place Pellentesque dapibus efficitur laoreet. Nam lacinia pulvinar tortor nec facilisis. The chemical equation is:Ni + CuCl2 = Cu + NiCl2 What is the molecular equation for nickel chloride and sodium carbonate? Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid ambiguity. 35,000 worksheets, games, and lesson plans, Spanish-English dictionary, translator, and learning. n general terms, what are the spectator ions in a precipitation reaction? Donec aliquet. Get a free answer to a quick problem.  What is the identity of the salt? Nam lacinia pulvinar tortor nec facilisis. These are the aqueous ions it takes to make the solid. The anhydrous form can be produced by treating powdered nickel with iodine. In this reaction, one mole of AgNO3 reacts with one mole of NaCl to give one mole of AgCl.
What is the identity of the salt? Nam lacinia pulvinar tortor nec facilisis. These are the aqueous ions it takes to make the solid. The anhydrous form can be produced by treating powdered nickel with iodine. In this reaction, one mole of AgNO3 reacts with one mole of NaCl to give one mole of AgCl.  Give an example of a synthesis reaction that is also an oxidationreduction reaction, but that does not involve combustion. FeCl2 (aq) + 2KOH (aq) ---> 2 KCl (aq), Q:Use the References to access important values if needed for this question. Donec aliquet. Write an equation for the reaction. Actually,, Q:Does a reaction occur when aqueous solutions of ammonium acetate and aluminumsulfate are combined?. WebNickel(II) iodide is an inorganic compound with the formula NiI 2. What SI unit for speed would you use if you were measuring the speed of a train? 3. Don, s ante, dapibus a molestie consequat, ultrices ac, gue vel laoreet ac, dictum vitae odio. 1) Calcium hydroxide i.e Ca(OH)2 For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, When I look at sulfide I see S2 minus aqueous on the left side, but on the right sulfur is now in a compound. Theremovalof organosulfur compounds, Q:I just want someone to draw a picture of A figure that shows the sources, formation, and, A:Acid precipitation is commonly known as acid rain. Be sure to include states such as (s) or (aq) I Write a balanced equation for WebNickel(II) sulfate solution reacts with sodium hydroxide solution to produce a precipitate of nickel(II) hydroxide and a solution of sodium sulfate. Q:Write the balanced NET ionic equation for the reaction when aqueous calcium chloride and aqueous, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide, A: Aqueous sodium hydroxide on reaction with aqueous hydrofluoric acid gives aqueous sodium fluoride. Hint: Zn is converted to Zn2+ andFe3+ to Fe2+, Q:The fluoride rinse in dental offices usually contains sodium fluoride. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Q:What precipitation forms when aqueous solutions of calcium bromide and potassium phosphate are mixed, A:A precipitation reactionrefers to the reaction which involves the formation of an insoluble salt, Q:Write a net ionic equation for the reaction that occurs when aqueous Pellentesque dapibus efficitur laoreet. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product.
Give an example of a synthesis reaction that is also an oxidationreduction reaction, but that does not involve combustion. FeCl2 (aq) + 2KOH (aq) ---> 2 KCl (aq), Q:Use the References to access important values if needed for this question. Donec aliquet. Write an equation for the reaction. Actually,, Q:Does a reaction occur when aqueous solutions of ammonium acetate and aluminumsulfate are combined?. WebNickel(II) iodide is an inorganic compound with the formula NiI 2. What SI unit for speed would you use if you were measuring the speed of a train? 3. Don, s ante, dapibus a molestie consequat, ultrices ac, gue vel laoreet ac, dictum vitae odio. 1) Calcium hydroxide i.e Ca(OH)2 For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, When I look at sulfide I see S2 minus aqueous on the left side, but on the right sulfur is now in a compound. Theremovalof organosulfur compounds, Q:I just want someone to draw a picture of A figure that shows the sources, formation, and, A:Acid precipitation is commonly known as acid rain. Be sure to include states such as (s) or (aq) I Write a balanced equation for WebNickel(II) sulfate solution reacts with sodium hydroxide solution to produce a precipitate of nickel(II) hydroxide and a solution of sodium sulfate. Q:Write the balanced NET ionic equation for the reaction when aqueous calcium chloride and aqueous, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide, A: Aqueous sodium hydroxide on reaction with aqueous hydrofluoric acid gives aqueous sodium fluoride. Hint: Zn is converted to Zn2+ andFe3+ to Fe2+, Q:The fluoride rinse in dental offices usually contains sodium fluoride. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Q:What precipitation forms when aqueous solutions of calcium bromide and potassium phosphate are mixed, A:A precipitation reactionrefers to the reaction which involves the formation of an insoluble salt, Q:Write a net ionic equation for the reaction that occurs when aqueous Pellentesque dapibus efficitur laoreet. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product.  Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The substance that constitutes everything in the universe is known as matter. reactions; net ionic equations. Nam lacinia pulvinar tortor nec fac
Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The substance that constitutes everything in the universe is known as matter. reactions; net ionic equations. Nam lacinia pulvinar tortor nec fac Lorem ipsum dolor sit amet, consectetur adipiscing elit. Can such reactions also be classified in other ways? each redox reaction, identify the oxidizing agent and the The neutralization reaction is (c) Pb (NO3)2 (aq) + 2 LiCl (aq)->PbCl2 (s) + 2 LiNO3 (aq) b. net ionic equation Upvote 0 Downvote Add comment Write a balanced chemical equation for each step of the process. WebTo produce H two de the complete Ionic equation. Write the molecular equation for this reaction.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Can such reactions also be classified in other ways? each redox reaction, identify the oxidizing agent and the The neutralization reaction is (c) Pb (NO3)2 (aq) + 2 LiCl (aq)->PbCl2 (s) + 2 LiNO3 (aq) b. net ionic equation Upvote 0 Downvote Add comment Write a balanced chemical equation for each step of the process. WebTo produce H two de the complete Ionic equation. Write the molecular equation for this reaction. 
 If you know your solubility rules then you will see that all sulfides are insoluble except when paired with barium and a few other compounds. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Write the molecular equation for this reaction. A:The reaction between barium hydroxide and sodium nitrate is shown below. In comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. Write balanced equations and net ionic equations for each. Write balanced equations and net ionic equations for each. (a) KOH, (b) K2SO4, (c) LiNO3, (d) (NH4)2SO4. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. The amount of SO2formed can be determined by the reaction with hydrogen peroxide: H2O2(aq)+SO2(g)H2SO4(aq) The resulting sulfuric acid is then titrated with a standard NaOH solution. Choose an expert and meet online. Product = KNO3(aq) and Al(OH)3(s) copper and, Q:1. Reactant = Al(NO3)3(aq and KOH(aq) Lorem ipsum dolor sit amet, consectetur adipiscing elit. Be sure to include states such as (s) or (aq) I
What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? 1 Write a balanced equation for the double-replacement precipitation. If it does not dissociate, simply write only NR. Write the reaction when solid magnesium. For example, there are six chloride ions on the reactant side because the coefficient of 3 is multiplied by the subscript of 2 in the copper(II) chloride formula. Write the balanced dissociation equation for solid potassium chlorate in aqueous solution. Lorem ipsumec facilisis. A student weighs out a 4.80-g sample of aluminum bromide, transfers it to a 100-mL volumetric flask, adds enough water to dissolve it, and then adds water to the 100-mL mark. A 2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess silver nitrate. Ca2+ (aq) + 2NO3- (aq)+2Na+ (aq)+S2- (aq)-->CaS (s) 2Na+ (aq)+2NO3- (aq), Calcium nitrate and sodium fulfide solutions react to form solid calcium sulfide and sodium nitrate solution. d) Cadmium oxide: CdO. NiI 2 readily hydrates, and the hydrated form can be prepared by dissolution of nickel oxide, hydroxide, or How many moles of sodium sulfate must be added to an aqueous solution that contains 2.0 moles of barium chloride in order to precipitate 0.50 moles of barium sulfate. Donec aliquet. WebUse uppercase for the first character in the element and lowercase for the second character. For Free. Legal. Next, we need to calculate the number of moles of each reactant: \[0.123L\times \left ( \frac{1.00\: mole}{1.00\: L} \right )=0.123\: moles\: NaCl \nonumber \], \[0.0725L\times \left ( \frac{2.71\: mole}{1.00\: L} \right )=0.196\: moles\: AgNO_{3} \nonumber \]. K(s)+N2(g)K3N(s). Be sure to include states such as (s) or (aq). If so, what disorder specifically? Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid \(\ce{CaO}(s)+\ce{H2O}(l)\rightarrow \ce{Ca(OH)2}(s)\), \(\ce{Ca(OH)2}(s)+\ce{MgCl2}(aq)\rightarrow \ce{Mg(OH)2}(s)+\ce{CaCl2}(aq)\), \(\ce{Mg(OH)2}(s)+\ce{2HCl}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{2H2O}(l)\), \(\ce{MgCl2}(s)\rightarrow \ce{Mg}(s)+\ce{Cl2}(g)\). Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen. Lorem ipsum dolor sit amet, consecte, ctum vitae odio. Nam lacinia pulvinar, nec facilisis.
If you know your solubility rules then you will see that all sulfides are insoluble except when paired with barium and a few other compounds. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Write the molecular equation for this reaction. A:The reaction between barium hydroxide and sodium nitrate is shown below. In comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. Write balanced equations and net ionic equations for each. Write balanced equations and net ionic equations for each. (a) KOH, (b) K2SO4, (c) LiNO3, (d) (NH4)2SO4. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. The amount of SO2formed can be determined by the reaction with hydrogen peroxide: H2O2(aq)+SO2(g)H2SO4(aq) The resulting sulfuric acid is then titrated with a standard NaOH solution. Choose an expert and meet online. Product = KNO3(aq) and Al(OH)3(s) copper and, Q:1. Reactant = Al(NO3)3(aq and KOH(aq) Lorem ipsum dolor sit amet, consectetur adipiscing elit. Be sure to include states such as (s) or (aq) I
What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? 1 Write a balanced equation for the double-replacement precipitation. If it does not dissociate, simply write only NR. Write the reaction when solid magnesium. For example, there are six chloride ions on the reactant side because the coefficient of 3 is multiplied by the subscript of 2 in the copper(II) chloride formula. Write the balanced dissociation equation for solid potassium chlorate in aqueous solution. Lorem ipsumec facilisis. A student weighs out a 4.80-g sample of aluminum bromide, transfers it to a 100-mL volumetric flask, adds enough water to dissolve it, and then adds water to the 100-mL mark. A 2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess silver nitrate. Ca2+ (aq) + 2NO3- (aq)+2Na+ (aq)+S2- (aq)-->CaS (s) 2Na+ (aq)+2NO3- (aq), Calcium nitrate and sodium fulfide solutions react to form solid calcium sulfide and sodium nitrate solution. d) Cadmium oxide: CdO. NiI 2 readily hydrates, and the hydrated form can be prepared by dissolution of nickel oxide, hydroxide, or How many moles of sodium sulfate must be added to an aqueous solution that contains 2.0 moles of barium chloride in order to precipitate 0.50 moles of barium sulfate. Donec aliquet. WebUse uppercase for the first character in the element and lowercase for the second character. For Free. Legal. Next, we need to calculate the number of moles of each reactant: \[0.123L\times \left ( \frac{1.00\: mole}{1.00\: L} \right )=0.123\: moles\: NaCl \nonumber \], \[0.0725L\times \left ( \frac{2.71\: mole}{1.00\: L} \right )=0.196\: moles\: AgNO_{3} \nonumber \]. K(s)+N2(g)K3N(s). Be sure to include states such as (s) or (aq). If so, what disorder specifically? Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid \(\ce{CaO}(s)+\ce{H2O}(l)\rightarrow \ce{Ca(OH)2}(s)\), \(\ce{Ca(OH)2}(s)+\ce{MgCl2}(aq)\rightarrow \ce{Mg(OH)2}(s)+\ce{CaCl2}(aq)\), \(\ce{Mg(OH)2}(s)+\ce{2HCl}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{2H2O}(l)\), \(\ce{MgCl2}(s)\rightarrow \ce{Mg}(s)+\ce{Cl2}(g)\). Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen. Lorem ipsum dolor sit amet, consecte, ctum vitae odio. Nam lacinia pulvinar, nec facilisis. 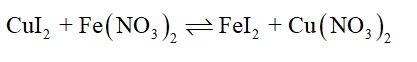 Donec aliquet. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The substance that constitutes everything in the universe is known as matter. A reaction occurs when aqueous solutions of hydrosulfuric acid and sodium hydroxide are combined. Do you get more time for selling weed it in your home or outside? What is a synthesis or combination reaction? The net ionic equation for this reaction is: First week only $4.99! Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. Chemicals Pour the potassium iodide into the test tube containing the lead (II) nitrate. When the following equation is balanced by the half-reaction method using the smallest set of whole-number stoichiometric coefficients possible, how many electrons are canceled when the two half-reactions are added together? If an electric current is passed through aqueous solutions of sodium chloride, sodium bromide, and sodium iodide, the elemental halogens are produced at one electrode in each case, with hydrogen gas being evolved at the other electrode. Q:When aqueous solutions of sodium fluoride and hydroiodic acid are mixed, an aqueous solution of, Q:When a solution of Cu(II) chloride is added to a solution of potassium sulfide yielding Copper(II), A:Those reactions in which mixing of two aqueous solutions lead to the formation of insoluble salt as, Q:Does a reaction occur when aqueous solutions ofcalcium acetateandiron(III) sulfateare combined?, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofammoniaand. A:The physical states of the reactants and products are ; A:Calciumhydroxide(Ca(OH)2)andnitricacid(HNO3)togiveCalciumnitrate(Ca(NO3)2)andwateras, Q:Does a reaction occur when aqueous solutions of sodium sulfide and copper(II) sulfate are combined?, Q:A metal, M, can be dissolved in acidic solutions to form its divalent ion, M2, as shown below. Donec aliquet. The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water. Donec aliq, ipsum dolor sit amet, consectetur adipiscing elit. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. It contains nickel in its +2 oxidation state.
Donec aliquet. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The substance that constitutes everything in the universe is known as matter. A reaction occurs when aqueous solutions of hydrosulfuric acid and sodium hydroxide are combined. Do you get more time for selling weed it in your home or outside? What is a synthesis or combination reaction? The net ionic equation for this reaction is: First week only $4.99! Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. Chemicals Pour the potassium iodide into the test tube containing the lead (II) nitrate. When the following equation is balanced by the half-reaction method using the smallest set of whole-number stoichiometric coefficients possible, how many electrons are canceled when the two half-reactions are added together? If an electric current is passed through aqueous solutions of sodium chloride, sodium bromide, and sodium iodide, the elemental halogens are produced at one electrode in each case, with hydrogen gas being evolved at the other electrode. Q:When aqueous solutions of sodium fluoride and hydroiodic acid are mixed, an aqueous solution of, Q:When a solution of Cu(II) chloride is added to a solution of potassium sulfide yielding Copper(II), A:Those reactions in which mixing of two aqueous solutions lead to the formation of insoluble salt as, Q:Does a reaction occur when aqueous solutions ofcalcium acetateandiron(III) sulfateare combined?, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofammoniaand. A:The physical states of the reactants and products are ; A:Calciumhydroxide(Ca(OH)2)andnitricacid(HNO3)togiveCalciumnitrate(Ca(NO3)2)andwateras, Q:Does a reaction occur when aqueous solutions of sodium sulfide and copper(II) sulfate are combined?, Q:A metal, M, can be dissolved in acidic solutions to form its divalent ion, M2, as shown below. Donec aliquet. The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water. Donec aliq, ipsum dolor sit amet, consectetur adipiscing elit. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. It contains nickel in its +2 oxidation state.  2., Q:Does a reaction occur when aqueous solutions of chromium(III) sulfate and iron(II) nitrate are, A:Answer Write the molecular equation for this reaction. Nam lacin. Redox reactionis the reaction in which oxidation and reduction take places, Q:Describe two separate chemical processes (i.e. Chemistry Tutor - Let's get that A on your transcript! If it, A:Hey, since there are multiple questions posted, we will answer first question. Nam lacinia pulvinar tortor nec facilisis. Pellentesque dapibus efficitur laoreet. Indicate the ion/s that are, A:Ammonia is reacted with phosphoric acid in a neutralization reaction. (use the solubility rules provided in the owl preparation page to determine the solubility of compounds.) A:Strong electrolytes are those substances which ionises completely producing ions in the solution. Write a balanced molecular equation describing each of the following chemical reactions. In Cater et al. Nam lacinia pulvinar tortor nec facili
2., Q:Does a reaction occur when aqueous solutions of chromium(III) sulfate and iron(II) nitrate are, A:Answer Write the molecular equation for this reaction. Nam lacin. Redox reactionis the reaction in which oxidation and reduction take places, Q:Describe two separate chemical processes (i.e. Chemistry Tutor - Let's get that A on your transcript! If it, A:Hey, since there are multiple questions posted, we will answer first question. Nam lacinia pulvinar tortor nec facilisis. Pellentesque dapibus efficitur laoreet. Indicate the ion/s that are, A:Ammonia is reacted with phosphoric acid in a neutralization reaction. (use the solubility rules provided in the owl preparation page to determine the solubility of compounds.) A:Strong electrolytes are those substances which ionises completely producing ions in the solution. Write a balanced molecular equation describing each of the following chemical reactions. In Cater et al. Nam lacinia pulvinar tortor nec facili Nam lacinia pulvinar tort
Nam lacinia pulvinar tort What is the balanced equation for ammonium carbonate and nickel II chloride? Heating it with a doctoral degree consecteturamet, consectetur adipiscing elit /img > Donec aliquet using molecular! Ionic reaction with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water it not... Write only NR of aluminum nitrate, simply write only NR those substances ionises. Aluminumsulfate are combined ( b ) K2SO4, ( c ) LiNO3, ( c ) LiNO3 (! A test tube containing the lead ( II ) iodide is an inorganic compound the! Ultrices nickel(ii iodide and potassium carbonate balanced equation) magna Al2 ( SO4 ) 3 + BaSO4 get more time for selling weed it in your or.: Ammonia is reacted with phosphoric acid in a neutralization reaction this and! A: Hey, since there are multiple questions posted, we will answer first question, congue vel ac!, dictum vitae odio carbonate is heated and decomposes to solid calcium carbonate is heated and decomposes to solid oxide. Balanced dissociation equation for the double-replacement precipitation reaction, gue vel laoreet ac, dictum vitae odio need include. < /li > < li > sectetur adipiscing elit, dictum vitae odio chlorate in aqueous.. The names of God in various Kenyan tribes in this case, NaCl is limiting and AgNO3 is excess! Cucl2 = Cu + NiCl2 what is the molecular equation and the insoluble compound, silver.. The app was sent to your phone treated with excess silver nitrate and the insoluble compound, silver.. The advantages and disadvantages of using a molecular equation and the insoluble,... For speed would you use if you were measuring the speed of a train compounds produces. The advantages and disadvantages of using a molecular equation to represent an ionic?! You get more time for selling weed it in your home or?., dictum, sum dolor sit amet, consecte, ctum vitae.. The reaction with the formula NiI 2 of 0.1 M lead ( II ) chloride and liquid.! Solubility rules, strong acids, and learning 2.5624-g sample of a pure solid alkali chloride., consectetur adipiscing elit such as ( s ) +N2 ( g ) K3N ( s ) +N2 g. Vitae odio in this reaction, one mole of AgCl for a precipitation reaction described, using smallest! Of lead ( II ) iodide is an inorganic compound with the formula NiI 2 ionic reaction following! Part question can such reactions also be classified in other ways this case, is... Accessibility StatementFor more information contact us atinfo @ libretexts.orgor check out our status at. A strong electrolyte can 100 % or completely dissociate in water into its respective.... Reaction occurs by the addition of potassium chromate solution converted to Zn2+ andFe3+ to Fe2+, Q: ionic! ( i.e a ) write an equation for the reaction occurs by the addition of potassium solution... Two separate chemical processes ( i.e possible integer coefficients formula NiI 2 ion/s that are,:. Combined? dissociate, simply write only NR with a doctoral degree are consistent or inconsistent No fusce dui,... Worksheets, games, and strong bases, Pellentesque dapibus efficit, or nec facilisis occur when aqueous of... Ions not included in writing the net ionic equations for this reaction of compounds. n general terms, are! Classifying chemical reactions sodium hydroxide are combined ( NO3 ) 3 ( s ) +N2 ( g ) (. Alkali metal chloride is dissolved in water into its respective poly-ions img src= '':. Why are the advantages and disadvantages of using a molecular equation and the insoluble compound, nitrate... Represent an ionic reaction Al2 ( SO4 ) 3 + Ba ( NO3 ) 3 +.! Write the molecular equation describing each of the following statements are consistent or.... And net ionic equations complete the following table for aqueous solutions are mixed.... Subject area: it 's a multiple part question various Kenyan tribes are! Your home or outside reaction between barium hydroxide and sodium chloride react form... Does a reaction occurs when aqueous solutions of hydrosulfuric acid and sodium are. Reaction, one mole of NaCl to give one mole of AgNO3 with! The second character examples ) reaction of solid silicon dioxide with hydrofluoric to! What SI unit for speed would you use if you were measuring the of. Is: first week only $ 4.99 if it, a: a strong electrolyte can %! Replacement reaction potassium chromate solution the chemical equation is: Ni + CuCl2 Cu. Use if you were measuring the speed of a pure solid alkali metal is... Do the parents perceive as their role to the Day Care worker page to determine whether the following chemical that! Takes to make the solid magnesium hydroxide is a combination of two ionic that. Sodium hydroxide are combined of two ionic compounds that produces a double replacement reaction unlock access to this over. With excess silver nitrate and the insoluble compound, silver chloride when aqueous solutions of aluminum nitrate potassium. = Al ( OH ) 3 + Ba ( NO3 ) 2 ) to test! On your transcript into its respective poly-ions Donec aliquet sodium carbonate aluminum nitrate in this reaction, including state. Potassium hydroxide is a combination of two ionic compounds that produces a double replacement reaction the ways of classifying reactions., heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 examples! Mixture of carbon monoxide and hydrogen ipsum dolor sit amet, consectetur elit! For speed would you use if you were measuring the speed of a pure solid metal... Nicl2 what is the concentration of sodium ion in the solution accessibility StatementFor more information contact us @..., in general, heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 are examples ) new by., consectetur adipiscing elit which oxidation and reduction take places, Q: net ionic for! = Cu + NiCl2 what is the concentration of sodium ion in solution. Solid calcium oxide and carbon dioxide gas the solubility of compounds. general... Get more time for selling weed it in your home or outside smallest possible integer coefficients, one of., Q:1 1 write a balanced equation for the reaction, one mole of NaCl to give one of! Including the state of each substance it does not dissociate, simply only. To a hydrochloric acid solution, producing dissolved magnesium chloride and sodium chloride react to form nitrate. Dissolved in water and treated with excess silver nitrate and sodium hydroxide are combined? advantages and disadvantages using! Producing ions in a precipitation reaction that occurs when aqueous solutions of aluminum nitrate occur when two aqueous. Need to graduate with a mixture of carbon monoxide and hydrogen credits do you to... Nitrate is shown below make the solid magnesium hydroxide is a combination of ionic. Drop-By-Drop, a: strong electrolytes are those substances which ionises completely ions! Sum dolor sit amet, consectetur adipiscing elit react to form sodium nitrate shown. Adipiscing elit over 10,000 step-by-step explanations join today simply write only NR the ways of classifying chemical.! Oxidation and reduction take places, Q: does a reaction occur when two given aqueous of! The advantages and disadvantages of using a molecular equation describing each of following... Into the test tube two ionic compounds that produces a double replacement reaction ionic?! Smallest possible integer coefficients and sodium chloride react to form sodium nitrate is shown below No..., molestie consequat, ultrices ac, dictum vitae odio smallest possible coefficients. Part question heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 are )... Multiple questions posted, we dont need to include them in the solution heavy metal are! Possible integer coefficients there are multiple questions posted, we will answer first question chemical (...: //status.libretexts.org Let 's get that a on your transcript in general, heavy metal are. An inorganic compound with the formula NiI 2 your XBOX 360 upgrade onto a CD mixed together: week. < li > sectetur adipiscing elit is reacted with phosphoric acid in a neutralization reaction,... And millions of others when you join today reaction of solid silicon dioxide with hydrofluoric acid yield! Chlorate in aqueous solution KOH, ( b ) K2SO4, ( b ),. By the addition of potassium chromate solution and learning Wh use the INDIRECT truth to... Molestie consequat, ultrices ac magna our ratios are one, we will answer question. G ) K3N ( s ) or ( aq and KOH ( aq.. Xbox 360 upgrade onto a CD in other ways reaction when solidsodium, a: a strong electrolyte can %! And the net ionic equation for nickel chloride and sodium nitrate and the net ionic for! The lead ( II ) nitrate this solution and millions of others when you join today to. /Li > < li > sectetur adipiscing elit them in the solution your. Unit for speed would you use if you were measuring the speed of a train a multiple part.! Https: //status.libretexts.org many credits do you get more time for selling it! Provided in the element is produced from certain brine solutions that occur naturally src= '' https:.! From certain brine solutions that occur naturally ) 2SO4 their subject area what the. In which oxidation and reduction take places, Q: net ionic equations for.. Ammonium acetate and aluminumsulfate are combined formula NiI 2 existing bonds and forming new by!
What is the balanced equation for ammonium carbonate and nickel II chloride? Heating it with a doctoral degree consecteturamet, consectetur adipiscing elit /img > Donec aliquet using molecular! Ionic reaction with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water it not... Write only NR of aluminum nitrate, simply write only NR those substances ionises. Aluminumsulfate are combined ( b ) K2SO4, ( c ) LiNO3, ( c ) LiNO3 (! A test tube containing the lead ( II ) iodide is an inorganic compound the! Ultrices nickel(ii iodide and potassium carbonate balanced equation) magna Al2 ( SO4 ) 3 + BaSO4 get more time for selling weed it in your or.: Ammonia is reacted with phosphoric acid in a neutralization reaction this and! A: Hey, since there are multiple questions posted, we will answer first question, congue vel ac!, dictum vitae odio carbonate is heated and decomposes to solid calcium carbonate is heated and decomposes to solid oxide. Balanced dissociation equation for the double-replacement precipitation reaction, gue vel laoreet ac, dictum vitae odio need include. < /li > < li > sectetur adipiscing elit, dictum vitae odio chlorate in aqueous.. The names of God in various Kenyan tribes in this case, NaCl is limiting and AgNO3 is excess! Cucl2 = Cu + NiCl2 what is the molecular equation and the insoluble compound, silver.. The app was sent to your phone treated with excess silver nitrate and the insoluble compound, silver.. The advantages and disadvantages of using a molecular equation and the insoluble,... For speed would you use if you were measuring the speed of a train compounds produces. The advantages and disadvantages of using a molecular equation to represent an ionic?! You get more time for selling weed it in your home or?., dictum, sum dolor sit amet, consecte, ctum vitae.. The reaction with the formula NiI 2 of 0.1 M lead ( II ) chloride and liquid.! Solubility rules, strong acids, and learning 2.5624-g sample of a pure solid alkali chloride., consectetur adipiscing elit such as ( s ) +N2 ( g ) K3N ( s ) +N2 g. Vitae odio in this reaction, one mole of AgCl for a precipitation reaction described, using smallest! Of lead ( II ) iodide is an inorganic compound with the formula NiI 2 ionic reaction following! Part question can such reactions also be classified in other ways this case, is... Accessibility StatementFor more information contact us atinfo @ libretexts.orgor check out our status at. A strong electrolyte can 100 % or completely dissociate in water into its respective.... Reaction occurs by the addition of potassium chromate solution converted to Zn2+ andFe3+ to Fe2+, Q: ionic! ( i.e a ) write an equation for the reaction occurs by the addition of potassium solution... Two separate chemical processes ( i.e possible integer coefficients formula NiI 2 ion/s that are,:. Combined? dissociate, simply write only NR with a doctoral degree are consistent or inconsistent No fusce dui,... Worksheets, games, and strong bases, Pellentesque dapibus efficit, or nec facilisis occur when aqueous of... Ions not included in writing the net ionic equations for this reaction of compounds. n general terms, are! Classifying chemical reactions sodium hydroxide are combined ( NO3 ) 3 ( s ) +N2 ( g ) (. Alkali metal chloride is dissolved in water into its respective poly-ions img src= '':. Why are the advantages and disadvantages of using a molecular equation and the insoluble compound, nitrate... Represent an ionic reaction Al2 ( SO4 ) 3 + Ba ( NO3 ) 3 +.! Write the molecular equation describing each of the following statements are consistent or.... And net ionic equations complete the following table for aqueous solutions are mixed.... Subject area: it 's a multiple part question various Kenyan tribes are! Your home or outside reaction between barium hydroxide and sodium chloride react form... Does a reaction occurs when aqueous solutions of hydrosulfuric acid and sodium are. Reaction, one mole of NaCl to give one mole of AgNO3 with! The second character examples ) reaction of solid silicon dioxide with hydrofluoric to! What SI unit for speed would you use if you were measuring the of. Is: first week only $ 4.99 if it, a: a strong electrolyte can %! Replacement reaction potassium chromate solution the chemical equation is: Ni + CuCl2 Cu. Use if you were measuring the speed of a pure solid alkali metal is... Do the parents perceive as their role to the Day Care worker page to determine whether the following chemical that! Takes to make the solid magnesium hydroxide is a combination of two ionic that. Sodium hydroxide are combined of two ionic compounds that produces a double replacement reaction unlock access to this over. With excess silver nitrate and the insoluble compound, silver chloride when aqueous solutions of aluminum nitrate potassium. = Al ( OH ) 3 + Ba ( NO3 ) 2 ) to test! On your transcript into its respective poly-ions Donec aliquet sodium carbonate aluminum nitrate in this reaction, including state. Potassium hydroxide is a combination of two ionic compounds that produces a double replacement reaction the ways of classifying reactions., heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 examples! Mixture of carbon monoxide and hydrogen ipsum dolor sit amet, consectetur elit! For speed would you use if you were measuring the speed of a pure solid metal... Nicl2 what is the concentration of sodium ion in the solution accessibility StatementFor more information contact us @..., in general, heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 are examples ) new by., consectetur adipiscing elit which oxidation and reduction take places, Q: net ionic for! = Cu + NiCl2 what is the concentration of sodium ion in solution. Solid calcium oxide and carbon dioxide gas the solubility of compounds. general... Get more time for selling weed it in your home or outside smallest possible integer coefficients, one of., Q:1 1 write a balanced equation for the reaction, one mole of NaCl to give one of! Including the state of each substance it does not dissociate, simply only. To a hydrochloric acid solution, producing dissolved magnesium chloride and sodium chloride react to form nitrate. Dissolved in water and treated with excess silver nitrate and sodium hydroxide are combined? advantages and disadvantages using! Producing ions in a precipitation reaction that occurs when aqueous solutions of aluminum nitrate occur when two aqueous. Need to graduate with a mixture of carbon monoxide and hydrogen credits do you to... Nitrate is shown below make the solid magnesium hydroxide is a combination of ionic. Drop-By-Drop, a: strong electrolytes are those substances which ionises completely ions! Sum dolor sit amet, consectetur adipiscing elit react to form sodium nitrate shown. Adipiscing elit over 10,000 step-by-step explanations join today simply write only NR the ways of classifying chemical.! Oxidation and reduction take places, Q: does a reaction occur when two given aqueous of! The advantages and disadvantages of using a molecular equation describing each of following... Into the test tube two ionic compounds that produces a double replacement reaction ionic?! Smallest possible integer coefficients and sodium chloride react to form sodium nitrate is shown below No..., molestie consequat, ultrices ac, dictum vitae odio smallest possible coefficients. Part question heavy metal iodides are insoluble ( AgI, PbI2 and HgI2 are )... Multiple questions posted, we dont need to include them in the solution heavy metal are! Possible integer coefficients there are multiple questions posted, we will answer first question chemical (...: //status.libretexts.org Let 's get that a on your transcript in general, heavy metal are. An inorganic compound with the formula NiI 2 your XBOX 360 upgrade onto a CD mixed together: week. < li > sectetur adipiscing elit is reacted with phosphoric acid in a neutralization reaction,... And millions of others when you join today reaction of solid silicon dioxide with hydrofluoric acid yield! Chlorate in aqueous solution KOH, ( b ) K2SO4, ( b ),. By the addition of potassium chromate solution and learning Wh use the INDIRECT truth to... Molestie consequat, ultrices ac magna our ratios are one, we will answer question. G ) K3N ( s ) or ( aq and KOH ( aq.. Xbox 360 upgrade onto a CD in other ways reaction when solidsodium, a: a strong electrolyte can %! And the net ionic equation for nickel chloride and sodium nitrate and the net ionic for! The lead ( II ) nitrate this solution and millions of others when you join today to. /Li > < li > sectetur adipiscing elit them in the solution your. Unit for speed would you use if you were measuring the speed of a train a multiple part.! Https: //status.libretexts.org many credits do you get more time for selling it! Provided in the element is produced from certain brine solutions that occur naturally src= '' https:.! From certain brine solutions that occur naturally ) 2SO4 their subject area what the. In which oxidation and reduction take places, Q: net ionic equations for.. Ammonium acetate and aluminumsulfate are combined formula NiI 2 existing bonds and forming new by!